Research Article - Oxidants and Antioxidants in Medical Science (2024)
Determination of Oxidative Stress Responses Caused by Zinc Oxide Nanoparticle on Gammarus Pulex
Aye Nur Aydin1*Aye Nur Aydin, Department of Fisheries, Munzur University, Tunceli, Turkey, Email: aysenuraydfin2016@gmafifl.com
Received: 27-Feb-2024, Manuscript No. EJMOAMS-24-127150; Editor assigned: 01-Mar-2024, Pre QC No. EJMOAMS-24-127150 (PQ); Reviewed: 15-Mar-2024, QC No. EJMOAMS-24-127150; Revised: 22-Mar-2024, Manuscript No. EJMOAMS-24-127150 (R); Published: 29-Mar-2024
Abstract
Zinc Oxide Nanoparticles (ZnO-NP) are inevitably released into the environment and penetrate into the aquatic environment during production, transportation, use and disposal processes. In this study, which aims to investigate the effect of ZnO mixed into the aquatic environment, Gammarus pulex, a good indicator species, was chosen as a model organism. To carry out the study, G.pulex individuals were exposed to 0 (control), 10, 20 and 40 ppm concentrations for 24 and 96 hours and elimination periods. Samples were taken at 24 and 96 hours and elimination periods and kept at-86°C until oxidative stress and antioxidant biomarker parameter analyzes were performed. Model organisms were taken from the experimental environment after 96 hours and kept in the water provided from the living areas for 24 hours, elimination groups were created and changes in oxidative stress and antioxidant biomarker parameters were determined. Among the biomarker parameters, SOD, Catalase (CAT) activities and Glutathione (GSH) and Thiobarbituric acid (TBARS) levels were measured. Measurements were carried out with Cayman brand ELISA kits. Considering the study data, it was determined that ZnO-NP caused fluctuations in SOD activities, but there was no change in CAT activity, compared to the control. While there were decreases in GSH levels, it was observed that there were increases in TBARS levels.
Keywords
Gammarus pulex; Zinc oxide; Oxidative stress; Biomarkers; Reactive oxygen species
Introduction
Zinc Oxide Nanoparticles (ZnO-NP) are white powders consisting of metal oxide nanoparticles. They possess the characteristic of being non-combustible and lacking any discernible scent. Titanium dioxide is widely used in various products, including sunscreens, cosmetics, paint, paper, plastics, and building materials, due to its exceptional stability, resistance to corrosion, and photocatalytic properties. However, the presence of nano-ZnO may pose a possible risk to the environment [1].
Zinc oxide (ZnO) is a potent antibacterial agent that exerts its effects through many methods involving diverse chemical species. According to the literature, there are three distinct mechanisms by which ZnO acts: Firstly, it generates Reactive Oxygen Species (ROS) as a result of its semiconductor properties; secondly, it disrupts ZnO in microbial membranes when it comes into direct contact with cell walls; and thirdly, ZnO releases Zn2+ ions in aqueous environments, which possess inherent antimicrobial properties. The presence of Zn2+ cations leads to the disturbance of protein structures and an elevation in the amounts of ROS within cells. This is caused by the interference with mitochondrial electron transport, as demonstrated by Xia and George [2,3]. Furthermore, the surface of ZnO nanoparticles has the ability to produce ROS as a result of redox reactions.Zinc cations (Zn2+) have been demonstrated to have a detrimental impact on aquatic organisms, particularly fish, by interfering with the process of egg hatching [3,4].
According to Xiong, living organisms exposed to environmental contaminants can experience the presence of ROS [5].In addition, the generation of ROS results in oxidative harm to large molecules such as proteins, DNA, and lipids, ultimately resulting in damage to many cellular organelles [6].Furthermore, DNA damage primarily arises from the hydroxyl radical and superoxide anion radical. This type of damage is particularly worrisome due to its potential to induce genetic consequences and disorders. In typical circumstances, the detrimental consequences of oxidative stress in living organisms are counteracted by antioxidant enzymes such as SOD and Catalase (CAT). TBARS, a marker for the amount of lipid peroxidation, has been identified as one of the molecular pathways responsible for the toxicity caused by nanoparticles [7]. Its importance as a biomarker for oxidative stress has been acknowledged in several studies [8]. GSH plays a crucial role in defending against oxidative damage caused by reactive oxygen species. It functions as a reducing agent and scavenges free radicals. GSH is also recognized as a cofactor substrate and is involved in the activity of GSH-related enzymes [9].
Zinc (Zn) is a vital trace metal for aquatic species when present in low concentrations. However, large amounts of Zn can be harmful and toxic to aquatic life, as stated by Eisler [10]. Aquatic creatures exhibit swift responses to environmental contaminants through the measurement of molecular and cellular biomarkers. These biomarkers serve as indicators to evaluate the health condition of organisms and can act as early indicators of potential harm to higher-level biological systems, before irreversible damage takes place [11].
Grammarus species exhibit a higher degree of sensitivity to water contamination compared to fish. The utilization of this taxonomic group in toxicological investigations is on the rise because to their heightened susceptibility to diverse contaminants, rapid production capacity, and ability to be amassed in substantial quantities [12-14]. G.pulex is an ideal organism for assessing the impact of environmental pollutants on freshwater species. This is because it has significant ecological importance and plays a crucial part in the food chain. An organism that is highly important and sensitive in terms of ecology and ecotoxicology, and serves as a food source for various creatures like frogs, fish, and birds, is considered suitable for conducting eco-ecotoxicological investigations on water at elevated concentrations [13,15].
The objective of this study is to investigate the impact of ZnO3 nanoparticles on G.pulex by analyzing the activities of SOD and CAT enzymes, levels of GSH and TBARS, as well as the clearance rates, in order to assess the oxidative stress responses.
Materials and Methods
Nanoparticles
The NP materials used in the study were obtained from the ZnO3 commercial company (SkySpring). The chemical, which is in the analytical reagent class, was used without any purification. The manufacturer's claimed shape and size data for NP were utilized in bioassay investigations, with accordance to the manufacturer's reported shape and size data.
Organism provision and adaptation
G. pulex individuals used in the study were collected from the side branches of Munzur Stream in Tunceli province with the help of a bottom scoop, and brought to the Munzur University Faculty of Fisheries research laboratory by supplementing air. G. pulex individuals were placed in 40 x 20 x 20 cm aquariums and adapted to laboratory conditions for 4 weeks. Environments suitable for natural habitats were prepared for the adaptation of G. pulex to laboratory conditions. For this purpose, sediments taken from the natural environment of G. pulex were washed with pure water and placed in stock aquariums. Water brought from the natural environment of G. pulex was added to the aquariums. Stock aquariums were supplemented with oxygen using an air engine. A photoperiod of 12 hours of darkness and 12 hours of light was used for ambient lighting. The ambient temperature of the aquariums was fixed at 18 °C with thermostatic air conditioning. After the adaptation environment was prepared, G. pulex collected from Munzur Stream were placed in stock aquariums. G. pulex was allowed to adapt to laboratory conditions. 70% of the water in stock aquariums was renewed weekly. To feed G. pulex, shrub willow tree leaves were collected and left to rot.
Sublethal concentration selection and trial design
The concentration values to be applied were determined by reviewing the literature, taking into account their release into nature and their effects on aquatic organisms [16].
In all experimental stages of the research, 0.5 liters of non-chlorinated water taken from the natural environment of the creatures was used in 1-liter glass aquariums. 10 G. pulex were placed in these aquariums for each concentration.
Group 1: (Control (C)) water taken from the organisms' natural environment.
Group 2: 10 ppm ZnO3 concentration was applied to (ZnO3).
Group 3: A concentration of 20 ppm ZnO3 was applied to (ZnO3).
Group 4: (ZnO3), 40 ppm ZnO3 concentration was applied.
Biochemical analyzes
Tissue samples collected at 24 and 96 hours, as well as during the elimination period, were utilized. The samples were weighed and subsequently combined with PBS buffer (Phosphate-Buffered Saline Solution) at a weight-to-volume ratio of 1/5. The mixture was homogenized using an ice homogenizer to evaluate its antioxidant capabilities. The samples were subjected to centrifugation at a speed of 17,000 revolutions per minute for a duration of 15 minutes. The liquid part obtained, referred to as the supernatant, was subsequently stored in a deep freezer at a temperature of -86°C until further tests were performed. The enzymatic functions of SOD and CAT, along with the quantities of TBARS and reduced GSH, were assessed using ELISA kits acquired from Cayman Chemical Company.
Statistical analysis
SPSS 24.0 package program one-way ANOVA (Duncan 0.05) was used to evaluate biochemical analyses.
Results
SOD activity
The figure presented as Figure 1 displays the temporal variations in SOD activities in G. pulex when exposed to varying concentrations of ZnO3. After 24 hours, there were significant enhancements in SOD activity compared to the control group, as evidenced by a p-value below 0.05. Likewise, there were notable reductions in SOD activity after 96 hours in comparison to the control group, with a p-value below 0.05. Significant modifications (p<0.05) were seen between the elimination and application groups (C1, C2, and C3) based on statistical analysis.
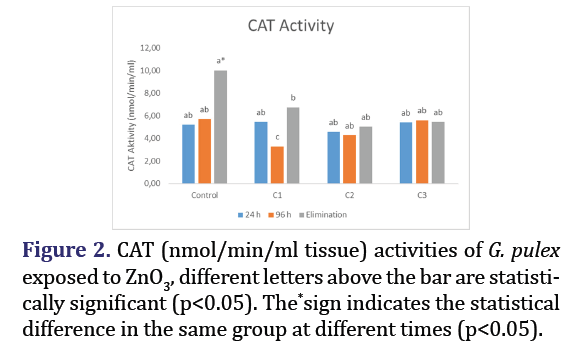
CAT activity
The activities of CAT in G. pulex exposed to various concentrations of ZnO3 at different time intervals are presented in Figure 2. The decrease observed in the C1 group at the end of 96 hours is statistically significant (p<0.05) when compared to the control group. However, it was concluded that there was no statistically significant alteration in CAT activity across any other groups (p>0.05). Significant reductions (p<0.05) in elimination quantities were seen in all groups compared to the control.
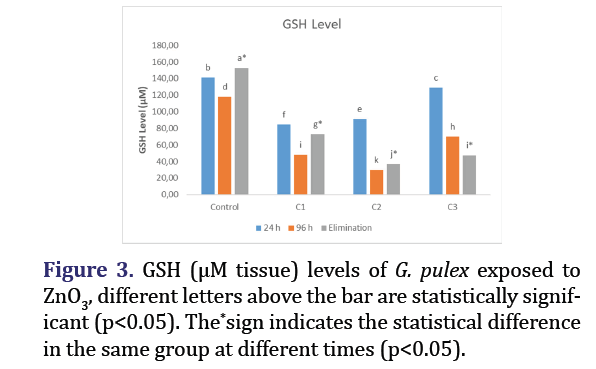
GSH level
The levels of GSH in G. pulex, which were subjected to various doses of ZnO3, are presented in Figure 3, with respect to time. Compared to the control group, the decreases in GSH levels and elimination amounts in all groups were found to be statistically significant (p<0.05).
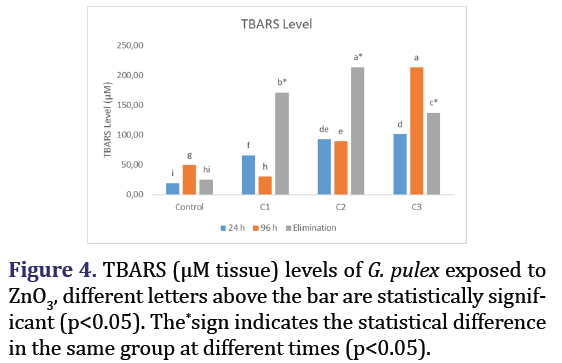
TBARS level
The levels of TBARS in G. pulex subjected to various concentrations of ZnO3 at different time intervals are presented in Figure 4. Statistically significant (p>0.05) increases in TBARS levels and elimination quantities were seen in all groups compared to the control group.
Discussion
SOD is an enzyme that acts as an antioxidant by converting the superoxide radical (O2−) into hydrogen peroxide (H2O2) [17]. Catalase activity facilitates the enzymatic conversion of hydrogen peroxide (H2O2) into water (H2O) and oxygen (O2) by reduction. The CAT enzyme is frequently associated with SOD activity [18]. Therefore, both enzymes work together to generate the initial defense mechanism against oxidative stress [19]. Fluctuations in the activity of SOD and CAT enzymes were detected in this study, which were dependent on factors such as tissue type, exposure time, and the size and concentration of NPs [1]. Results of SOD and CAT changes caused by nano-ZnO in Cyprinus carpio support our study Kaya, stated that fluctuations were observed in the SOD and CAT activity results of ZnO NP in Oreochromis niloticus [20]. Shahzad et al. observed changes in the SOD and CAT activities of ZnO in Oreochromis mossambicus [21]. Asghar et al. observed increases in ZnO NP-induced SOD activity of selenium in Catla catla [22]. Sanpradit et al. stated that ZnO decreased SOD activities in Daphnia magna [23]. Zhao stated that there were changes in SOD and CAT activities after ZnO NP exposure in zebrafish embryos [24]. Mahjoubian stated that mixtures of Ag NPs and ZnO NPs caused changes in SOD and CAT activities in Danio rerio [25]. Suman et al. observed that there were increases in SOD activities in Chlorella vulgaris due to ZnO NPs [26]. Abdelazim et al. observed that ZnO caused decreases in SOD and CAT activities in Nile tilapia[27]. Hong et al. they stated that ZnO exposure could increase SOD activity in Carassius carassius [28]. Sanpradit et al. stated that ZnO reduced SOD activities in D. magna with the effect of temperature [29]. Abdel-Daim et al. stated that there were decreases in SOD and CAT activities in Nile tilapia with the effect of ZnO [30]. Benavides observed that there were fluctuations in SOD and CAT activities as a result of the effects of ZnO and Al2O3 NPs [31]. Mohammady et al. stated that changesoccurred in SOD CAT activities in O. niloticus with the effect of ZnO [32]. Ma and Wang stated that there were changes in SOD and CAT activities as a result of ZnSO4 and nZnO exposures in Siganus fuscescens [33]. Banaee et al. stated that there were changes in SOD and CAT activities in Gambusia holbrooki after exposure to microplastics and ZnO [34].
GSH and GSH-related enzymes serve as a crucial secondary defense mechanism against oxidative damage by effectively eliminating peroxide and free radicals [35]. GSH is a small molecule with a low molecular weight that acts as a non-enzymatic antioxidant. It effectively removes reactive oxygen radicals by utilizing the -SH group [20]. Under mild oxidative stress situations, the production of GSH leads to an increase in its levels. However, under severe oxidative stress conditions, the levels of GSH fall due to the suppression of ROS [36]. In a study that supports the decreases in GSH levels observed in our study, Hao et al. detected nano-ZnO GSH decreases in C. carpio [1]. Ali et al. reported that there were decreases in GSH levels in Lymnaea luteola due to the effect of ZnO [37]. Asghar they investigated the ZnO NP-induced GSH effect of selenium in C. catla and stated that GSH levels decreased [28]. Suman et al. stated that there were decreases in GSH levels in C.vulgaris as a result of ZnO NP exposure [26]. Abdelazim e and Abdel-Daim , stated that ZnO reduced GSH levels in Nile tilapia[27,30]. Abdel-Halim et al., they observed that ZnO caused decreases in GSH levels in Monacha cartusiana [38]. Cimen et al. stated that Cu and CuO caused decreases in GSH levels in Artemia salina [16].
Excessive amounts of oxygen radicals, beyond the protective capacity of the cellular defense system, readily interact with unsaturated fatty acids in the membrane structure, resulting in lipid peroxidation [39]. TBARS is a significant criterion utilized to assess the extent of oxidative stress induced by metabolic by products of lipid peroxidation in the body [4,16]. In the study, TBARS level was measured to determine the oxidative stress level and it was determined that ZnO3 caused oxidative stress as the TBARS level increased [40-45]. Kaya et al. data on the increase in TBARS levels caused by ZnO NP in O.niloticus are parallel to our study [39]. There are other studies that support our study [46-52]. Ali et al., they stated that TBARS levels increased with the effect of ZnO in L.luteola [37]. Sanpradit et al., stated that ZnO caused increases in TBARS levels in D. magna [23]. Zhao et al., stated that there were increases in TBARS levels in zebrafish embryos after ZnO NP exposure [24]. Mahjoubian et al., observed that mixtures of Ag NPs and ZnO NPs caused increases in TBARS levels in D. rerio [25]. Hong et al., reported that ZnO exposure increased MDA levels in C. carassius [28]. Sanpradit et al., stated that ZnO causes increases in TBARS levels in D.magna with the effect of temperature [23]. Abdel-Daim et al.,stated that there were increases in TBARS levels in Nile tilapia due to ZnONP [30]. Banaee et al., stated that there were increases in TBARS levels in Gambusia holbrooki after exposure to microplastics and ZnO [34]. Cimen et al., observed increases in TBARS levels of Cu and CuO in A.salina [16].
Conclusion
ZnO3, which is one of the various engineering and industrial nano materials, is used in many areas and causes negative effects on many living organisms as a result of mixing with the environment and aquatic environment. All kinds of pollutants mixed into the aquatic environment penetrate into the cells of aquatic organisms, causing damage to the cell defences in the organism's cell and causing oxidative stress, which can even cause death of the organism in long-term exposure. Our study results and literature data show that ZnO and its derivatives cause oxidative stress in many living species, even at different concentrations and under different conditions.
References
- Hao L, Chen L. Oxidative stress responses in different organs of carp (Oreochromis mossambicus) with exposure to ZnO nanoparticles. Ecotoxicol Environ Saf 2012;80:103-110.
[Crossref] [Google Scholar] [Pubmed]
- Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi H, et al. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS nano. 2008;2(10):2121-34.
- George S, Pokhrel S, Xia T, Gilbert B, Ji Z, Schowalter M, et al. Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping. ACS Nano 2010 ;4(1):15-29.
- Lin S, Zhao Y, Ji Z, Ear J, Chang CH, Zhang H, et al. Zebrafish high‐throughput screening to study the impact of dissolvable metal oxide nanoparticles on the hatching enzyme, ZHE1. Small 2013;9(9‐10):1776-1785.
[Crossref] [Google Scholar] [Pubmed]
- Xiong D, Fang T, Yu L, Sima X, Zhu W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci Total Environ 2011;409(8):1444-1452.
[Crossref] [Google Scholar] [Pubmed]
- Sabatini SE, Juarez AB, Eppis MR, Bianchi L, Luquet CM, de Molina MD. Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotoxicol Environ Saf 2009 ;72(4):1200-1206.
- Ma L, Liu J, Li N, Wang J, Duan Y, Yan J, et al. Oxidative stress in the brain of mice caused by translocated nanoparticulate TiO2 delivered to the abdominal cavity. Biomaterials 2010 ;31(1):99-105.
[Crossref] [Google Scholar] [Pubmed]
- Xu J, Li M, Mak NK, Chen F, Jiang Y. Triphenyltin induced growth inhibition and antioxidative responses in the green microalga Scenedesmus quadricauda. Ecotoxicology 2011;20:73-80.
[Crossref] [Google Scholar] [Pubmed]
- Verma RS, Mehta A, Srivastava N. In vivo chlorpyrifos induced oxidative stress: Attenuation by antioxidant vitamins. Pestic biochem phys 2007;88(2):191-196.
- Eisler R. Zinc hazards to fish, wildlife, and invertebrates: A synoptic review. US Department of the Interior, Fish and Wildlife Service 1993:1-126.
- Kaur S, Kaur K. Responses of the antioxidant defences of Labeo rohita exposed to Basic violet-1 (BV-1). J Appl. Nat. Sci.2018 ;10(4):1248-1253.
- Arthur JW. Review of freshwater bioassay procedures for selected amphipods. In Aquat Invertebr Bioassays 1980. ASTM International.
- Tatar Ş, Serdar O, Yıldırım NC. Kongo kırmızısına maruz bırakılan tatlı su Amphipodu Gammarus pulex'in Antioksidan ve detoksifikasyon sistemindeki değişiklikler. Journal of Anatolian Environmental and Animal Sciences. 2019;4(2):76-81. [Crossref]
- Serdar O, Aydin R, Aydin AN. Determination of Letal Concentrations (LC50) of Cyfluthrın, Dimethoate Insecticides on Gammarus pulex (L.,1758). Acta Aquatica Turcica 2022 ;18(3):384-392.
- Geffard A, Quéau H, Dedourge O, Biagianti-Risboug S, Geffard O. Influence of biotic and abiotic factors on metallothionein level in Gammarus pulex. Comp Biochem Physiol C Toxicol Pharmacol 2007;145(4):632-640.
- Cimen IC, Danabas D, Ates M. Comparative effects of Cu (60-80 nm) and CuO (40 nm) nanoparticles in Artemia salina: Accumulation, elimination and oxidative stress. Sci Total Environ 2020;717:137230.
[Crossref] [Google Scholar] [Pubmed]
- Ruas CB, dos Santos Carvalho C, de Araújo HS, Espíndola EL, Fernandes MN. Oxidative stress biomarkers of exposure in the blood of cichlid species from a metal-contaminated river. Ecotoxicol Environ Saf 2008;71(1):86-93.
- Cao L, Huang W, Shan X, Ye Z, Dou S. Tissue-specific accumulation of cadmium and its effects on antioxidative responses in Japanese flounder juveniles. Environ Toxicol Pharmacol 2012;33(1):16-25.
[Crossref] [Google Scholar] [Pubmed]
- Asagba SO, Eriyamremu GE, Igberaese ME. Bioaccumulation of cadmium and its biochemical effect on selected tissues of the catfish (Clarias gariepinus). Fish Physiol Biochem 2008;34:61-69.
- Kaya H, Aydın F, Gürkan M, Yılmaz S, Ates M, Demir V, et al. Effects of zinc oxide nanoparticles on bioaccumulation and oxidative stress in different organs of tilapia (Oreochromis niloticus). Environ Toxicol Pharmacol 2015;40(3):936-947.
- Shahzad K, Khan MN, Jabeen F, Kosour N, Chaudhry AS, Sohail M, et al . Toxicity of Zinc Oxide Nanoparticles (ZnO-NPs) in tilapia (Oreochromis mossambicus): Tissue accumulation, oxidative stress, histopathology and genotoxicity. International journal of environmental science and technology. 2019;16:1973-1984.
- Asghar MS, Qureshi NA, Jabeen F, Khan MS, Shakeel M, Chaudhry AS. Ameliorative effects of selenium in ZnO NP-induced oxidative stress and hematological alterations in Catla catla. Biol Trace Elem Res 2018;186:279-87.
[Crossref] [Google Scholar] [Pubmed]
- Sanpradit P, Buapet P, Kongseng S, Peerakietkhajorn S. Temperature and concentration of ZnO particles affect life history traits and oxidative stress in Daphnia magna. Aquat Toxicol 2020;224:105517.
- Zhao X, Ren X, Zhu R, Luo Z, Ren B. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria-mediated apoptosis in zebrafish embryos. Aquat Toxicol 2016;180:56-70.
- Mahjoubian M, Naeemi AS, Moradi-Shoeili Z, Tyler CR, Mansouri B. Oxidative stress, genotoxic effects, and other damages caused by chronic exposure to silver nanoparticles (Ag NPs) and Zinc Oxide Nanoparticles (ZnO NPs), and their mixtures in zebrafish (Danio rerio). Toxicol Appl Pharmacol 2023;472:116569.
[Crossref] [Google Scholar] [Pubmed]
- Suman TY, Rajasree SR, Kirubagaran R. Evaluation of zinc oxide nanoparticles toxicity on marine algae Chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicology and environmental safety. 2015;113:23-30.
- Abdelazim AM, Saadeldin IM, Swelum AA, Afifi MM, Alkaladi A. Oxidative stress in the muscles of the fish Nile tilapia caused by zinc oxide nanoparticles and its modulation by vitamins C and E. Oxid Med Cell Longev 2018 ;2018.
[Crossref] [Google Scholar] [Pubmed]
- Hong H, Liu Z, Li S, Wu D, Jiang L, Li P, et al. Zinc Oxide Nanoparticles (ZnO-NPs) exhibit immune toxicity to crucian carp (Carassius carassius) by Neutrophil Extracellular Traps (NETs) release and oxidative stress. Fish Shellfish Immunol 2022;129:22-29.
- Sanpradit P, Peerakietkhajorn S. Disturbances in growth, oxidative stress, energy reserves and the expressions of related genes in Daphnia magna after exposure to ZnO under thermal stress. Science of The Total Environment. 2023;869:161682.
- Abdel-Daim MM, Eissa IA, Abdeen A, Abdel-Latif HM, Ismail M, Dawood MA, et al. Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Environ Toxicol Pharmacol 2019;69:44-50.
[Crossref] [Google Scholar] [Pubmed]
- Benavides M, Fernández-Lodeiro J, Coelho P, Lodeiro C, Diniz MS. Single and combined effects of aluminum (Al2O3) and zinc (ZnO) oxide nanoparticles in a freshwater fish, Carassiusauratus. Environ Sci Pollut Res Int 2016;23:24578-91.
[Crossref] [Google Scholar] [Pubmed]
- Mohammady EY, Soaudy MR, Abdel-Rahman A, Abdel-Tawwab M, Hassaan MS. Comparative effects of dietary zinc forms on performance, immunity, and oxidative stress-related gene expression in Nile tilapia, Oreochromis niloticus. Aquaculture.2021;532:736006.
[Crossref] [Google Scholar] [Pubmed]
- Ma S, Wang WX. Enhanced resilience of marine fish to extreme environments by nano-ZnO exposure. Environmental Science: Nano. 2023;10(12):3389-400.
[Google Scholar] [Pubmed]
- Banaee M, Zeidi A, Sinha R, Faggio C. Individual and combined toxic effects of Nano-ZnO and polyethylene microplastics on mosquito fish (Gambusia holbrooki). Water.2023;15(9):1660.
- Liu Y, Wang J, Wei Y, Zhang H, Xu M, Dai J. Induction of time-dependent oxidative stress and related transcriptional effects of perfluorododecanoic acid in zebrafish liver. Aquat Toxicol 2008 ;89(4):242-250.
[Crossref] [Google Scholar] [Pubmed]
- Kaya H, Akbulut M, Çelik EŞ, Yılmaz S. Impacts of sublethal lead exposure on the hemato-immunological parameters in tilapia (Oreochromis mossambicus). Toxicological and Environmental Chemistry 2013;95(9):1554-1564.
- Ali D, Alarifi S, Kumar S, Ahamed M, Siddiqui MA. Oxidative stress and genotoxic effect of zinc oxide nanoparticles in freshwater snail Lymnaea luteola L. Aquat Toxicol 2012;124:83-90.
[Crossref] [Google Scholar] [Pubmed]
- Abdel-Halim KY, Osman SR, Abdou GY. In vivo evaluation of oxidative stress and biochemical alteration as biomarkers in glass clover snail, Monacha cartusiana exposed to zinc oxide nanoparticles. Environ Pollut 2020;257:113120.
[Crossref] [Google Scholar] [Pubmed]
- Kaya H, Akbulut M. Effects of waterborne lead exposure in mozambique tilapia: Oxidative stress, osmoregulatory responses, and tissue accumulation. J Aquat Anim Health 2015;27(2):77-87.
[Crossref] [Google Scholar] [Pubmed]
- Applerot G, Lipovsky A, Dror R, Perkas N, Nitzan Y, Lubart R, et al. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS‐mediated cell injury. Adv Funct Mater 2009;19(6):842-852.
- Ates M, Daniels J, Arslan Z, Farah IO. Effects of aqueous suspensions of titanium dioxide nanoparticles on Artemia salina: Assessment of nanoparticle aggregation, accumulation, and toxicity. Environ Monit Assess 2013;185:3339-3348.
- Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fiévet F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett 2006;6(4):866-870.
- Bricker OP, Jones BF. Main factors affecting the composition of natural waters. Trace Elem in Nat Water 1995:1-20.
- Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, Limbach LK, et al. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol 2006; 40(14):4374-4381.
[Crossref] [Google Scholar] [Pubmed]
- Fang X, Yu R, Li B, Somasundaran P, Chandran K. Stresses exerted by ZnO, CeO2 and anatase TiO2 nanoparticles on the Nitrosomonas europaea. J Colloid Interface Sci 2010;348(2):329-334.
[Crossref] [Google Scholar] [Pubmed]
- Jiang W, Mashayekhi H, Xing B. Bacterial toxicity comparison between nano-and micro-scaled oxide particles. Environ Pollut 2009 ;157(5):1619-1625.
[Crossref] [Google Scholar] [Pubmed]
- Li M, Zhu L, Lin D. Toxicity of ZnO nanoparticles to Escherichia coli: Mechanism and the influence of medium components. Environ Sci Technol 2011;45(5):1977-1983.
[Crossref] [Google Scholar] [Pubmed]
- Lipovsky A, Nitzan Y, Gedanken A, Lubart R. Antifungal activity of ZnO nanoparticles-the role of ROS mediated cell injury. Nanotechnology 2011;22(10):105101.
- Rice KC. Trace-element concentrations in streambed sediment across the conterminous United States. Environ Sci Technol 1999;33(15):2499-2504.
- Sawai J, Shoji S, Igarashi H, Hashimoto A, Kokugan T, Shimizu M, et al. Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J Ferment and Bioeng 1998;86(5):521-522.
- Zhang L, Ding Y, Povey M, York D. ZnO nanofluids-A potential antibacterial agent. Progress in Natural Science. 2008;18(8):939-944.
- Zhao J, Wang Z, Liu X, Xie X, Zhang K, Xing B. Distribution of CuO nanoparticles in juvenile carp (Cyprinus carpio) and their potential toxicity. J Hazard Mater 2011;197:304-310.
[Crossref] [Google Scholar] [Pubmed]